PMCF Evaluation Reports
How to successfully create a PMCF Report which is Medical Device Regulation (MDR) compliant
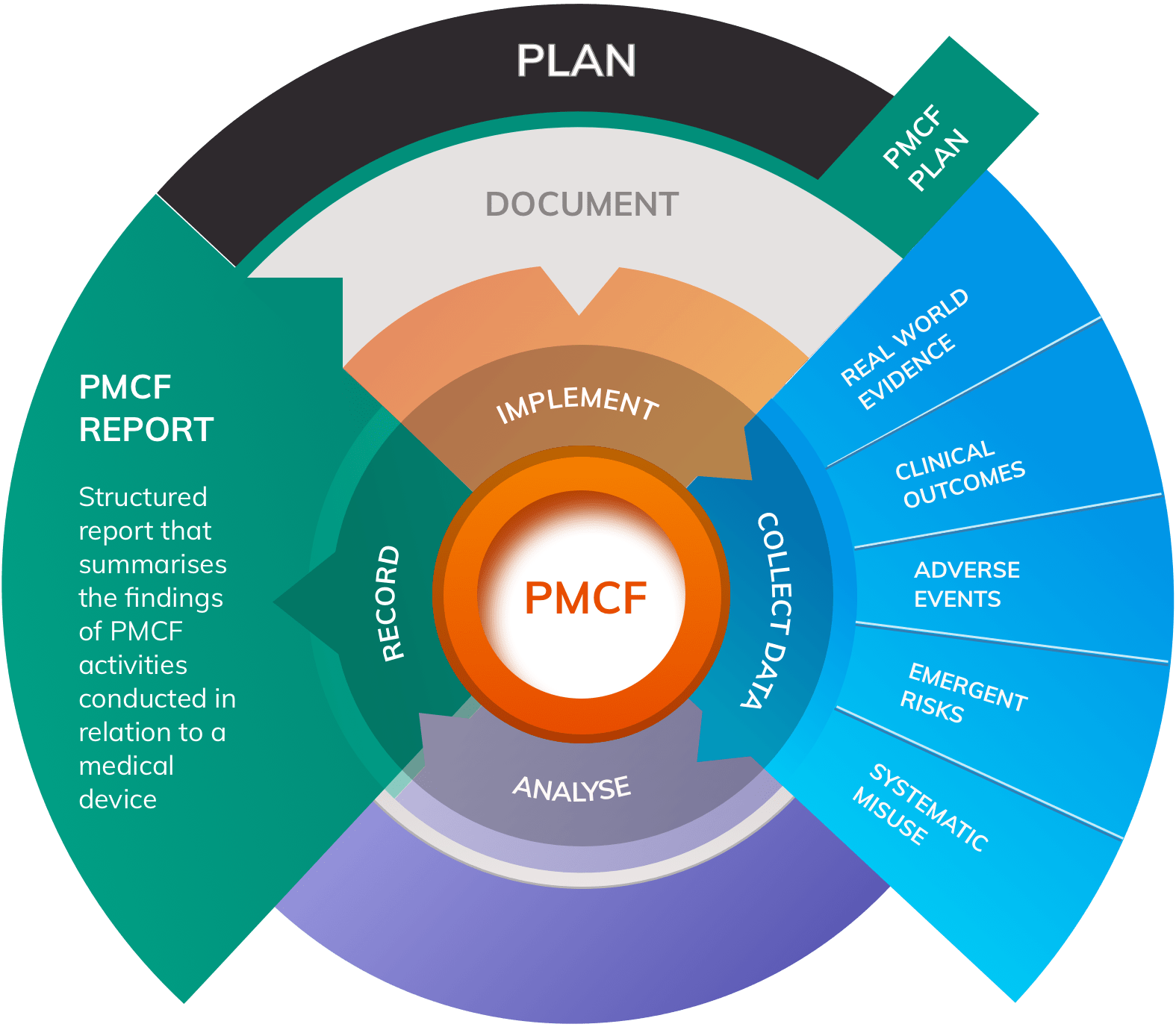
What is a PMCF Report?
A Post-Market Clinical Follow-up (PMCF) Report (also known as a PMCF Evaluation Report) is a structured report that summarises the findings of PMCF activities conducted in relation to a medical device. The Medical Device Regulation - MDR 2017/745 - significantly elevates the importance of PMCF compared to its level of prominence under the predecessor legislation, the Medical Device Directive MDD 93/42/EC.
Detailed rules for PMCF under the MDR are set out in Annex XIV Part B. All PMCF must be conducted according to a written PMCF plan, submitted as one component of the technical documentation needed for regulatory scrutiny along with the device itself.
Periodically, findings from PMCF must be summarised in a PMCF Report, with the contents of the Report forming an important input to the Clinical Evaluation Report (CER) for the device.
What should the PMCF Report contain?
Although MDR Annex XIV Part B contains detailed guidance for structuring a PMCF Plan, it does not go into similar detail for the requirements of the contents of the PMCF Report. This can lead to manufacturers feeling unsure as to what to include in the report.
The EU Commission produces a range of MedDev guidance documents intended to help medical device manufacturers meet regulatory requirements. One of these documents - MedDev 2.12/2 rev 2 - focuses on PMCF activities and gives some detail about requirements for PMCF Reports. MedDev 2.12/2 rev 2 has not been updated to reflect changes introduced by the MDR and still references Essential Requirements and other elements that were applicable to the MDD.
Fortunately, the MDCG Guidelines are updated with much greater regularity. MDCG 2020-8 contains detailed guidance on how to structure a PMCF Evaluation Report. While the MDCG Guidelines are not mandatory, alignment with them generally represents an accepted approach.
PMCF objectives should focus on areas such as:
- Adverse events
- Side-effect frequency and severity
- Systematic misuse of the product
- Safety and performance of the device in routine use
Therefore, data relating to each of these areas generated through PMCF studies can be summarised under the appropriate heading in the PMCF Report.
The report should also contain a summary of methods used to generate the data, and information about any method alternations that may be required for future PMCF activities. An assessment should also be made as to whether PMCF activities have been sufficient to address the full range of objectives specified in the plan.
What skills are necessary to write an MDR compliant PMCF Report?
The MDR as an entity draws heavily upon requirements for the production and interpretation of clinical data. An ability to critically appraise, interpret, and apply the findings of clinical evidence is essential in working with PMCF data, as is an ability to understand and appraise the methods by which data has been generated.
Medical professionals with suitable training in the field are very well positioned to write PMCF Reports and to work with many other aspects of MDR compliance that require the handling of clinical evidence. Mantra Systems has a team of in-house medical writers who are all ex-clinicians. This blend of regulatory and medical expertise means we are ideally positioned to assist with the development of PMCF Plans and Reports. Contact Us for a free consultation to explore your requirements in full.


