Anyone who’s worked on a Clinical Evaluation knows the drill by now: you’re deep into the narrative, you’ve built a clear clinical picture, the literature is supporting your claims, and then you hit the State of the Art (SOTA) section.
It’s one of the most deceptively complex parts of the Clinical Evaluation. The SOTA section, at its core, is about establishing the clinical context of your device. You’re identifying technologies, treatments, and medical devices that are comparable or similar to yours and demonstrating how your device measures up against them. In theory, this sounds straightforward, but here’s where things get complicated. While you’re focused on proving your device stands up to clinical scrutiny, regulators are equally interested in what you’re not including, and more importantly, why.
The Real Challenge: What You Leave Out Matters Just As Much
SOTA exclusions aren’t just a box-ticking exercise, they’re a true test of your reasoning and your ability to defend your choices with confidence. If you exclude devices, alternative technologies, or treatment options that seem relevant without a solid justification in place, the regulators will notice. It’s not enough to say, “This device isn’t comparable”, you need to explain why. Is it the indication? The mechanism of action? The patient population? Is there insufficient clinical evidence? Every exclusion must be logical, traceable, and transparent.
Why This Keeps Coming Up
This isn’t just a one-off issue, as it’s seen across many submissions. Most teams feel confident about what they’ve included in their SOTA, but the rationale for exclusions often ends up vague, underdeveloped, or missing entirely. The truth is, SOTA requires interpretation, and that’s exactly why it’s so frequently dissected. If a seemingly relevant comparator or treatment is left out without a strong explanation, it stands out.
Regulators don’t just want to see what you included, they want to understand why you didn’t include something else. Without that clarity, they may assume the exclusion was unintentional or that your review wasn’t comprehensive. In short, it keeps coming up because exclusions are easy to challenge and hard to justify unless you’ve done the thinking and documented it clearly.
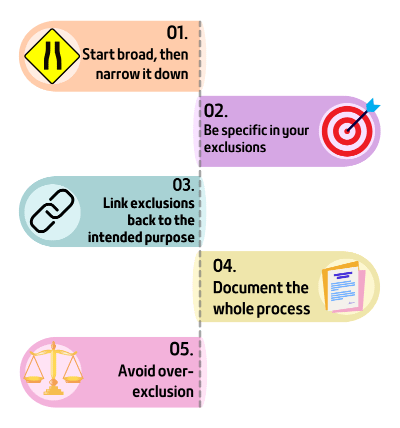
1. Start with a broad landscape, then narrow it down
Don’t begin with what you want to exclude; start with everything that could be relevant. List out all comparable technologies, treatments, and devices, even those you know won’t make the final cut. From there, document your rationale for keeping or excluding each one.
Tip: MedDev 2.7/1 rev 4 requires the use of carefully formulated inclusion / exclusion criteria, augmented by an objective, non-biased, systematic screening and review process such as PICO (patient characteristics, type of intervention, control, and outcome queries), or PRISMA (The Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
2. Be specific in your exclusions
Vague justifications are one of the fastest ways to undermine an otherwise strong Clinical Evaluation. A sentence like “This technology was excluded due to differences in application” is almost guaranteed to prompt questions. Instead, give clear, defensible reasons tied to your device’s design, function, and clinical use. Examples of strong justifications:
- Article reporting outcomes or clinical scenarios entirely unrelated to the subject matter.
- Not related to the intended purpose of the subject device. For example, an article focused on wound closure techniques would be an inappropriate focus when the subject matter of a review is the safety or performance of a hip implant.
- The literature contains unsubstantiated opinions
Tip: If you’re unsure how specific to be, err on the side of more. Specificity signals competence and confidence.
3. Link exclusions back to the device’s intended purpose
The intended purpose isn’t just regulatory terminology, it’s your compass. Every inclusion or exclusion should align with it. If something is excluded because it falls outside your indication, say so. If your device is single-use and another is reusable, that difference might be clinically meaningful.
Tip: Write your intended purpose in plain language and keep it visible during writing and editing. Use it as a live reference point when making decisions on what to include or exclude.
4. Document the process and justification (not just the outcome)
A polished summary is important, but so is the trail you took to get there. Regulators don’t just want to see the final result; they want to know you followed a systematic process to get there.
It may be helpful to use exclusion codes to codify reasons for exclusions (which may include, for example, “unrelated topic” or “article not available in English,” etc).
Tip: Save your search strings, selection criteria, and full extractions of literature from your databases. Include a short methods section describing how you determine exclusions. This small step can prevent a lot of back-and-forth during review.
5. Avoid over-exclusion
While it’s important to justify exclusions, it’s equally important not to exclude too many devices or treatments, as an overly narrow SOTA can look suspicious to regulators. Try to keep the scope broad, including all potentially relevant comparators, and only exclude those with a well-supported rationale.
Tip: Aim for a balance. Make sure your exclusions are justified, but ensure that your SOTA still includes a reasonable number of relevant sources, where deemed appropriate. It demonstrates thoroughness and strengthens your argument for your device’s clinical positioning.
Final Thought: Clarity and Transparency Will Always Be Your Strongest Defence
SOTA exclusions will likely remain one of the more complex aspects of a Clinical Evaluation submission. However, with a well-structured approach, the right tools, and a strong focus on clinical reasoning, you can turn this challenge into an opportunity. Clarity in the rationale for exclusions can enhance the strength of your submission by demonstrating thoroughness, transparency, and attention to detail.
By articulating your exclusions in a manner that’s easy to follow and backed by solid evidence, you make it easier for regulators to see your reasoning, leading to smoother reviews and fewer questions along the way. Navigating exclusions doesn’t have to be daunting, but it does require precision, thorough documentation, and a clear understanding of both clinical and regulatory expectations.
If you’re feeling uncertain about your SOTA exclusions or finding it difficult to manage the comparator landscape, don’t hesitate to reach out to Mantra Systems. We offer tailored support to guide you through the process, ensuring your Clinical Evaluation is both comprehensive and compliant. Let us help you get it right the first time.